Innovative Solutions for the Healthcare Industry
Forian provides innovative software solutions, proprietary data, and predictive analytics to optimize the operational, clinical, and financial performance of our healthcare customers.
The Engine of New Insights: The Forian Data Factory
With state-of-the-art ability to ingest, process, and deliver advanced solutions, we create quality, connected data in a secure and privacy-compliant method — for a new standard of analytics, insights, and product delivery.
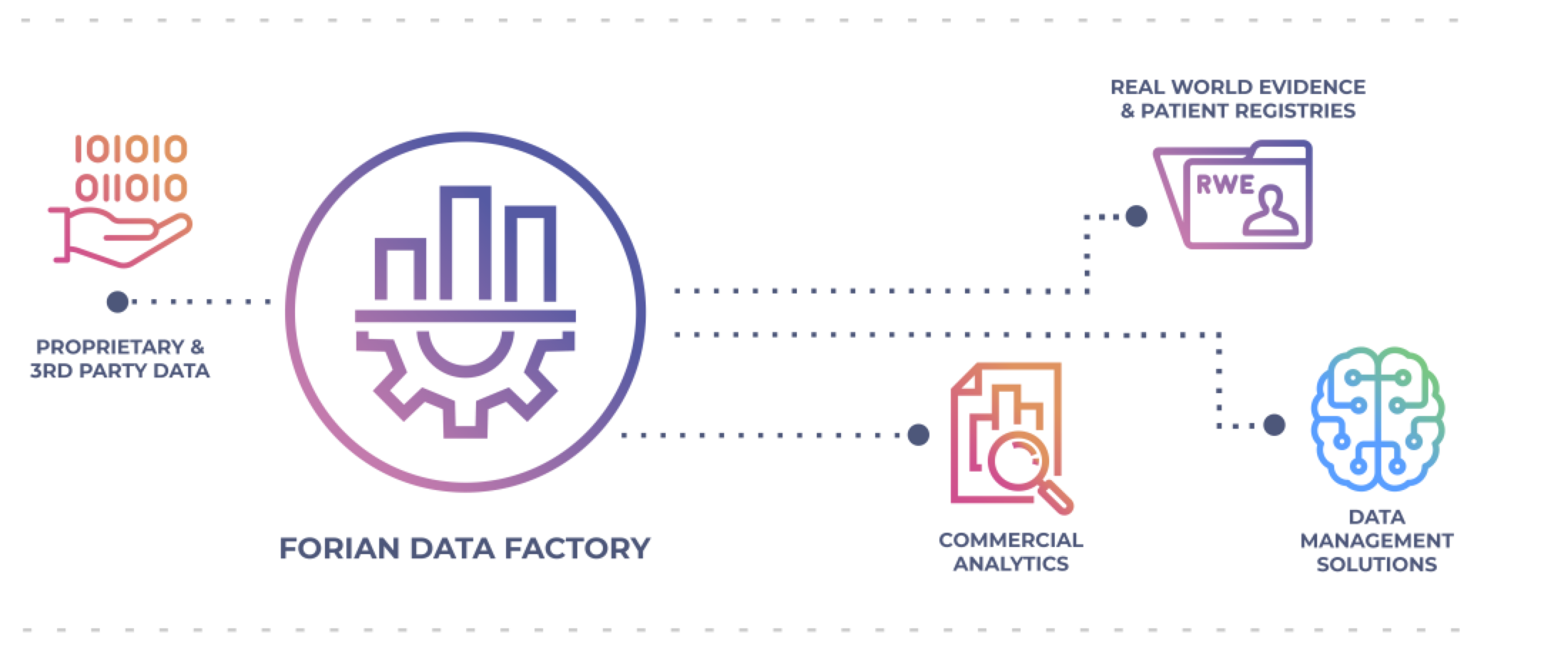
Commercial Analytics
Comprehensive proprietary data, coupled with advanced analytics, to improve business performance by maximizing customer understanding, delivering product knowledge, and tracking market dynamics over time.
Learn more >
Patient Registries
Leveraging the power of Real World Data (RWD) through custom-built patient registries, Patient-Reported Outcomes (PROs), and Health Economics and Outcomes Research (HEOR).
Learn more >
Real World Evidence (RWE)
Contextualizing clinical evidence in real world settings to derive actionable insights and optimize operational, clinical, and financial performance for life sciences, providers, and payers.
Learn more >
Data Management Solutions
Cutting-edge data science technology to efficiently organize, normalize, standardize, integrate, enhance, and publish complex consumable transactional information — all at scale — that is capable of generating targeted, unique, and actionable market-leading insights.
Learn more >

About Forian
Forian provides innovative software solutions, proprietary data, and predictive analytics to optimize the operational, clinical, and financial performance of our healthcare customers.
Navigation
© 2023 Forian Inc.
Privacy Policy Terms of Use

